|
Dr. Ellie L. Uzunova |
|
Institute of General and Inorganic Chemistry |
|
3d elements - Transition metal oxides. |
|
Research Topics |
|
© 2021-2026 All rights reserved |
|
Transition metal oxides. Our DFT studies are focused on the oxide clusters of the 3d elements. The interaction of molecular oxygen, for example, with transition metal centers leads to the formation of end-on η1-complex (superoxo- form), side-on η2-complex (peroxo- form) in which the O-O bond is weakened, or η0-complex in which O2 is dissociated to form a dioxide according to the following scheme: O2 + M ↔ O2(adsorbed)M ↔ O2–(adsorbed)M+ ↔ O22–(adsorbed)M2+ ↔ MOO ↔ M(O2) ↔ MO2 Density functional studies have proved reliable for the clusters MOn (n=1-4; M=Sc-Zn), the ordering of states corresponding to low-lying energy minima was confirmed by coupled-cluster calculations. The studies of neutral metal nitrosyls – particularly those of cobalt and nickel revealed closely spaced local minima. The linear CuNO lies close to the dissociation limit (in singlet state – above it), while the 1Σ+ and 3Σ+ states of linear CoNO form a conical intersection point on the potential energy surface (PES). The ground state of CoNO is 3A’. Copper nitrosyl is of lower thermodynamic stability than cobalt nitrosyl. A triplet ground state 3A” with bent configuration is predicted by B3LYP, confirmed by CAS MP2, and a very close-lying singlet state 1A’ is found with nearly identical geometry. For CuNO the triplet and singlet PES do not cross, but are separated by a small energy gap.
|
|
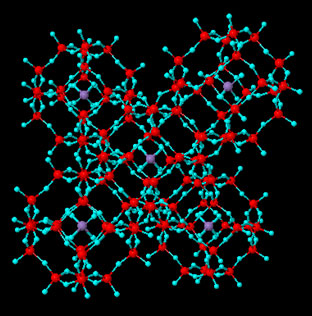
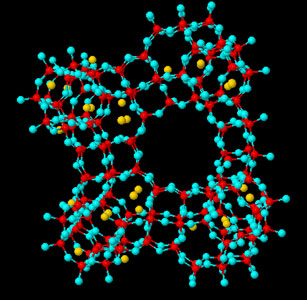
Zeolites, Zeolite-Related Materials and Molecular Sieves Zeolites are microporous aluminosilicate materials with special properties. The micropores are a structural feature; they consist of cavities and channels which are strictly ordered along the crystal. The primary building units are TO4 tetrahedra, connected via corner-sharing; each apical oxygen atom is shared between two adjacent tetrahedra. Though high-silica zeolites possess higher thermal stability, entirely siliceous structures like silicalite are not common. The central tetrahedral atom can be substituted: by B, Fe, Cr, Sb, As, Ga (replacing Al) or Ge, Ti, Zr, Hf (replacing Si). The orientation of the tetrahedra is not fixed and the angle T-O-T varies in a relatively broad range, which permits the formation of cage-like polyhedra. Some of these larger building blocks are common for a number of zeolite structures and are denoted as secondary building units. We have focused our research on double-rings as zeolite structural fragments, as they are thermodynamically stable and D4R (double four-membered rings of oxygen-bridged silicon and aluminum atoms) even with Si/Al substitution, have been isolated as individual compounds. D6R (double six-membered rings of oxygen-bridged silicon and aluminum atoms) are a secondary building unit in numerous zeolites. Here, we show structural models of FAU (cubic) and EMT (hexagonal).
|
|
FAU zeolite |
|
EMT zeolite |