|
Dr. Ellie L. Uzunova |
|
Institute of General and Inorganic Chemistry |
|
Recent Research Highlights |
|
© 2021-2026 All rights reserved |
|
The local minima on the singlet triplet and the quintet potential energy surfaces (PES) of cobalt and copper mononitrosyls have been examined by DFT with the B3LYP functional and by Complete Active Space methods (CASSCF and CAS MP2). The quintet states are separated from the singlet and triplet states by a high energy gap for both CoNO and CuNO, however the triplet and the singlet PES lie close to each other. The ground state of CoNO is 3A’; two local minima were found in singlet states: linear, 1Σ+, and side-on configuration, 1A1. The crossing of the singlet and triplet PES of CoNO is a conical intersection; the geometry of the bound state is linear, with elongated N-O bond. The activation energy for the spin-forbidden transition 3A’ → 1Σ+ is estimated to be 154.7 kJ mol‑1 (Figure 1). Copper nitrosyl is of lower thermodynamic stability than cobalt nitrosyl. A triplet ground state 3A” with bent configuration is predicted by B3LYP and confirmed by CAS MP2. A very close-lying singlet state 1A’ is found with nearly identical geometry. The triplet and singlet PES do not cross, but are separated by a small energy gap (Figure 2).
|


|
Transition metal cations in zeolites and related molecular-sieve structures; interactions with small molecules (di- and triatomic)
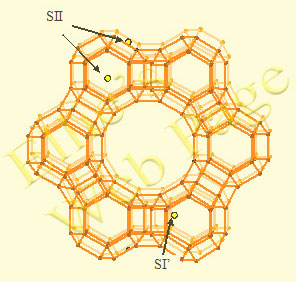
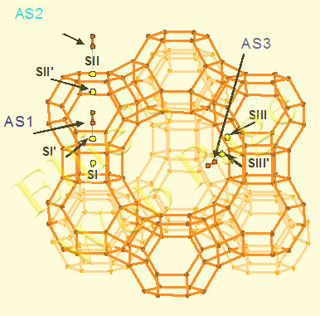
Zeolites and zeolite-related molecular sieve materials possess special properties: they have low framework density, large void volume, consisting of regularly ordered cavities and channels, and act as ion-exchangers. The micropores are an element of the structure and their apertures are strictly defined. They are comparable to the size of molecules and are able to allow only certain reactants or adsorbates to enter the channels, thus acting as “molecular sieves”. Though a strict distinction cannot be drawn, usually the term zeolite is used for Si and Al as tetrahedral atoms; a wide number of micropore materials, structurally related to zeolites and containing Ge, Ti, Zr at the place of Si, or B, Fe, Cr at the place of Al, are denoted as zeolite-related molecular sieves. In addition, structure analogs of zeolites can be built by alternation of Al and P at tetrahedral sites (ALPO’s) or Si, Al and P (SAPO’s). The presence of atoms with lower valency at tetrahedral sites assigns a negative charge of the framework, which is compensated by an extraframework cation or a proton. Common extraframework cations are the alkaline and alkaline-earth cations; they are readily exchangeable. Special interest deserves the exchange with transition-metal cations, as they create active site for redox reactions. The proton exchanged zeolites bear Brønsted acidity. The most important applications of zeolites in catalysis are due to their acidic properties – the presence of Brønsted and Lewis acid sites. Their strength and number can be tailored for use in heterogeneous catalysis. Zeolites and related materials in their acid form can be either used directly as catalysts, or as carriers of other active components. A typical feature is the shape selectivity, as products which fit the size of the pores can be formed. The presence of transition metal centers enables processes of hydrogenation and oxidation. Most of the heterogeneous catalytic oxidation reactions are known to proceed via the Mars-van Krevelen mechanism, according to which the re-oxidation of the transition metal center is the rate-determining step and the metal-oxygen bond strength is a crucial factor for the catalyst's activity. Copper-exchanged zeolites are an alternative to noble metal catalysts for the deNOx process. While the most extensively studied system is Cu-ZSM-5 and its high activity remains unchallenged, other structures and compositions are still of interest. We have focused our present study to Cu and Co exchanged FAU zeolites. A number of extraframework sites are available in FAU and EMT, of which most important are the sites denoted below. |
|
Figure 1. 3D and 2D representation of triplet and singlet PES of CoNO as a function of the internal coordinates RCo-N and Ð CoNO with RNO fixed at the value of the global minimum of PES, calculated by B3LYP. All local minima and the linear transition state 3Σ+ are denoted.
|
|
The challenge in the synthesis of tailored molecular sieves were extra-large pore materials. Zeolite structures have a beauty of their own: ALPO-5 with 12T rings of aperture 7.3 Å and VPI-5 with 12T rings of aperture 12.7 Å. |
|
Schematic representation of the FAU zeolite framework with extraframework cation adsorption sites denoted: AS1 (at site SI’), AS2 (at site SII) and AS3 (at site SIII’). The framework oxygen atoms are not shown; they lie near the center of line segments, connecting T-atoms. |



|
Figure 1. 3D representation of the triplet and the singlet PES of CuNO, calculated by B3LYP with all local minima denoted. |

