
|
Brief CV
Graduate Studies: Msc., Faculty of Chemistry, University of Sofia “St. Kliment Ochridski” PhD thesis: 1993, “Reactivity of High-Dispersity Binary Spinel Cobaltites of Ni, Cu and Zn.” 1994-1995: Post-Doctoral stay at the Center of Surface Science and Catalysis, Catholic University of Leuven, Belgium. 1996-2001: Research Associate in the Institute of General and Inorganic Chemistry, Bulgarian Academy of Sciences. 2001 till present: Associate Research Professor at the Institute of General and Inorganic Chemistry, Bulgarian Academy of Sciences.
Fields of Interest: Theoretical Inorganic Chemistry, Chemical Dynamics, DFT, Zeolites, Transition metal oxides, Catalysis. |
|
Transition Metal Oxides; Adsorption on Transition Metal Cation Centers. Our studies are focused on the oxide clusters of the 3d elements and we apply electronic structure methods: density functional theory, coupled cluster method, natural orbital analysis. The interaction of molecular oxygen, for example, with transition metal centers leads to the formation of end-on η1-complex (superoxo- form), side-on η2-complex (peroxo- form) in which the O-O bond is weakened, or η0-complex in which O2 is dissociated to form a dioxide according to the following scheme: O2 + M ↔ O2(adsorbed)M ↔ O2–(adsorbed)M+ ↔ O22–(adsorbed)M2+ ↔ MOO ↔ M(O2) ↔ MO2 The studies of neutral metal nitrosyls – particularly those of cobalt and nickel – revealed closely spaced local minima. The linear CuNO lies close to the dissociation limit (in singlet state – above it), while the 1Σ+ and 3Σ+ states of linear CoNO form a conical intersection point on the potential energy surface (PES). Adsorption and catalysis on transition metal extraframework cations in zeolites is examined by applying the ONIOM method. The active site is treated at the highest applicable level of theory; the PM6 method or molecular mechanics is used for the active site environment and the remaining part of the framework. |
|
“Cation binding of Li(I), Na(I) and Zn(II) to cobalt and iron sulphide clusters – electronic structure study” E. L. Uzunova Phys. Chem. Chem. Phys., 2022, 24, 20228-20238.
“Cation pair formation in copper and palladium exchanged MFI zeolite frameworks – a theoretical study” E. L. Uzunova Phys. Chem. Chem. Phys., 2019, 21, 14786-14798.
“Pathways of selective catalytic CO2 two-step reduction on di-iron, di-cobalt and iron-cobalt disulfide carbonyls – an electronic structure study ” E. L. Uzunova Catal. Sci. Technol., 2019, 9, 1039-1047.
“Theoretical Study of Nitrogen Dioxide and Nitric Oxide Co-Adsorption and DeNOx Reaction on Cu-SAPO-34 and Cu-SSZ-13 in Presence of Brønsted Acid Sites” E. L. Uzunova Molecular Catalysis 2018, 447, 47-55.
“Adsorption of phosphates and phosphoric acid in zeolite clinoptilolite: Electronic structure study” E. L. Uzunova, H. Mikosch Microporous and Mesoporous Materials 2016, 232, 119.
“A theoretical study of nitric oxide adsorption and dissociation on copper-exchanged zeolites SSZ-13 and SAPO-34: the impact of framework acid–base properties” E. L. Uzunova, H. Mikosch Phys. Chem. Chem. Phys., 2016, 18, 11233.
“CO2 Conversion to Methanol on Cu(I) Oxide Nanolayers and Clusters: Electronic Structure Insight into the Reaction Mechanism ” E. L. Uzunova, N. Seriani, H. Mikosch Phys. Chem. Chem. Phys., 2015, 17, 11088.
“Electronic, magnetic structure and water splitting reactivity of the iron-sulfur dimers and their hexacarbonyl complexes: A density functional study” E. L. Uzunova, H. Mikosch J. Chem. Phys., 2014, 141, 044307. “Electronic Structure and Reactivity in Water Splitting of the Iron Oxide Dimers and their Hexacarbonyls: a Density Functional Study” E. L. Uzunova, H. Mikosch J. Chem. Phys., 2014, 140, 024303. “Adsorption and Activation of Ethene in Transition Metal Exchanged Zeolite Clinoptilolite: a Density Functional Study” E. L. Uzunova, H. Mikosch ACS Catal. 2013, 3, 2759. “Cation Site Preference in Zeolite Clinoptilolite: A Density Functional Study” E. L. Uzunova, H. Mikosch Microporous and Mesoporous Materials 2013, 177, 113. “Electronic Structure and Reactivity of Cobalt Oxide Dimers and their Hexacarbonyl Complexes: a Density Functional Study” E. L. Uzunova, H. Mikosch J. Phys. Chem. A, 2012, 116, 3295 “Density Functional Study of Copper-Exchanged Zeolites and Related Microporous Materials. Adsorption of Nitrosyls” E. L. Uzunova, G. Nikolov H. Mikosch Int. J. Quant. Chem., 2013, 113, 723. “Density Functional Study of the Stable Oxidation States and the Binding of Oxygen in MO4 Clusters of the 3d Elements” E. L. Uzunova J. Phys. Chem. A, 2011, 115, 10665
“Electronic Structure of Trioxide, Oxoperoxide, Oxosuperoxide, and Ozonide Clusters of the 3d Elements: Density Functional Theory Study” E. L. Uzunova J. Phys. Chem. A, 2011, 115, 1320 “Intersystem Crossings of the Triplet and Singlet States in Cobalt and Copper Mononitrosyls” E. L. Uzunova J. Phys. Chem. A, 2009, 113, 11266 “Electronic Structure of Oxide, Peroxide and Superoxide Clusters of the 3d elements: a Comparative Density Functional Study” E. L. Uzunova, G. Nikolov H. Mikosch J. Chem. Phys., 2008, 128, 094307 “Theoretical Study of Transition Metal Cation Exchanged Zeolites: Interaction with NO” E. L. Uzunova, H. Mikosch, et al J. Mol. Struct. (THEOCHEM) 2009, 912, 88 “Application of Hybrid Functionals to the Modeling of NO Adsorption on Cu-SAPO-34 and Co-SAPO-34: A Periodic DFT Study” E. L. Uzunova, F. Göltl, G. Kresse, J. Hafner J. Phys. Chem. C, 2009, 113, 5274 “Adsorption of NO on Cu-SAPO-34 and Co-SAPO-34: A Periodic DFT Study” E. L. Uzunova, H. Mikosch, et al J. Phys. Chem. C, 2008, 112, 2632 “Interaction of Molecular Nitrogen and Oxygen with Extraframework Cations in Zeolites with Double Six-Membered Rings of Oxygen-Bridged Silicon and Aluminum Atoms: A DFT Study” E. L. Uzunova, G. Nikolov H. Mikosch J. Phys. Chem. B, 2005, 109, 11119 “Electronic Structure and Stability of Double Six-Membered Rings of Oxygen-Bridged Silicon and Aluminum Atoms Related to Cation Site Occupancy in FAU Zeolites: a DFT Study” E. L. Uzunova, H. Mikosch J. Phys. Chem. B, 2004, 108, 6981 “Vibrational Modes of Double Six-Member Rings of Oxygen-Bridged Silicon and Aluminum Atoms in Zeolites: a DFT Study” E. L. Uzunova, G. Nikolov H. Mikosch J. Phys. Chem. B, 2004, 108, 13200 “Manganese, Iron, Cobalt and Nickel Oxo-, Peroxo- and Superoxo-Clusters: A Density Functional Theory Study” E. L. Uzunova, G. Nikolov, H. Mikosch ChemPhysChem 5 (2004) 192-201 “Electronic Structure and Vibrational Modes of Cobalt Oxide Clusters CoOn and their Monoanions” E. L. Uzunova, G. Nikolov, H. Mikosch J. Phys. Chem. A, 2002, 106, 4104
For updated information see link. |

|
The triplet and the singlet PES of CuNO, calculated by B3LYP with all local minima denoted. |


|
Ethene adsorption and activation in zeolite clinoptilolite, studied by DFT with ONIOM |
|
Transition metal oxides have potential application as solar fuel cell materials. Iron and cobalt oxides have been subject of numerous studies. We have examined the ability of diiron dioxide and diiron disulfide hexacarbonyls to split water. The reaction mechanism on the singlet and triplet PES was traced by DFT and the activation energy for each elementary step was calculated. UV-VIS light absorption by the complexes was examined by time-dependent DFT, and reveal the possibility to activate the water splitting reaction by visible light irradiation. The cupri-oxide, Cu2O, recently attracted interest as a photo-catalyst for CO2 reduction. The surface stabilization of copper(I) oxide nanoclusters and nanolayers occurs via formation of Cu-Cu dimers and larger assemblies with Cu-Cu bonds. The DFT studies on the reaction mechanism of carbon dioxide reduction to methanol using dissociated water as a source of hydrogen on a Cu2O nanolayer and finite nano-clusters point to the formation of a stable carboxyl intermediate. Formic acid and formaldehyde are produced in the next elementary steps. They are strongly bound to the surface via hydrogen bonds, which favours further reduction instead of desorption. The nature of the active sites is revealed, namely the role of surface copper dimers, which are present on the Cu2O(001) surface and in the nanoclusters of size Cu32O16 and Cu14O7. |
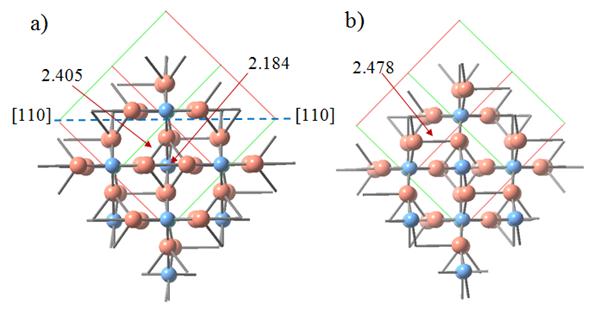
|
a) The Cu2O (001) copper-terminated surface with copper surface dimers and subsurface dimers denoted. b) The Cu2O (001) oxygen-terminated surface with copper surface dimers denoted. Cu atoms are red circles, O atoms – blue circles. |
|
Primary Address: Institute of General and Inorganic Chemistry Bulgarian Academy of Sciences Sofia 1113, BULGARIA |
|
To contact me: |
|
Fax: +(359 2) 870 5024 E-mail: ellie.uzunova@gmail.com |